Improving the patient experience in diagnosing a deadly liver disease
“NIMBLE HAS THE POTENTIAL TO GALVANIZE THE ENTIRE FIELD IN OUR PURSUIT OF AN ACCURATE, SCALABLE, NON-INVASIVE TOOL FOR THE DIAGNOSIS AND STAGING OF NASH.”

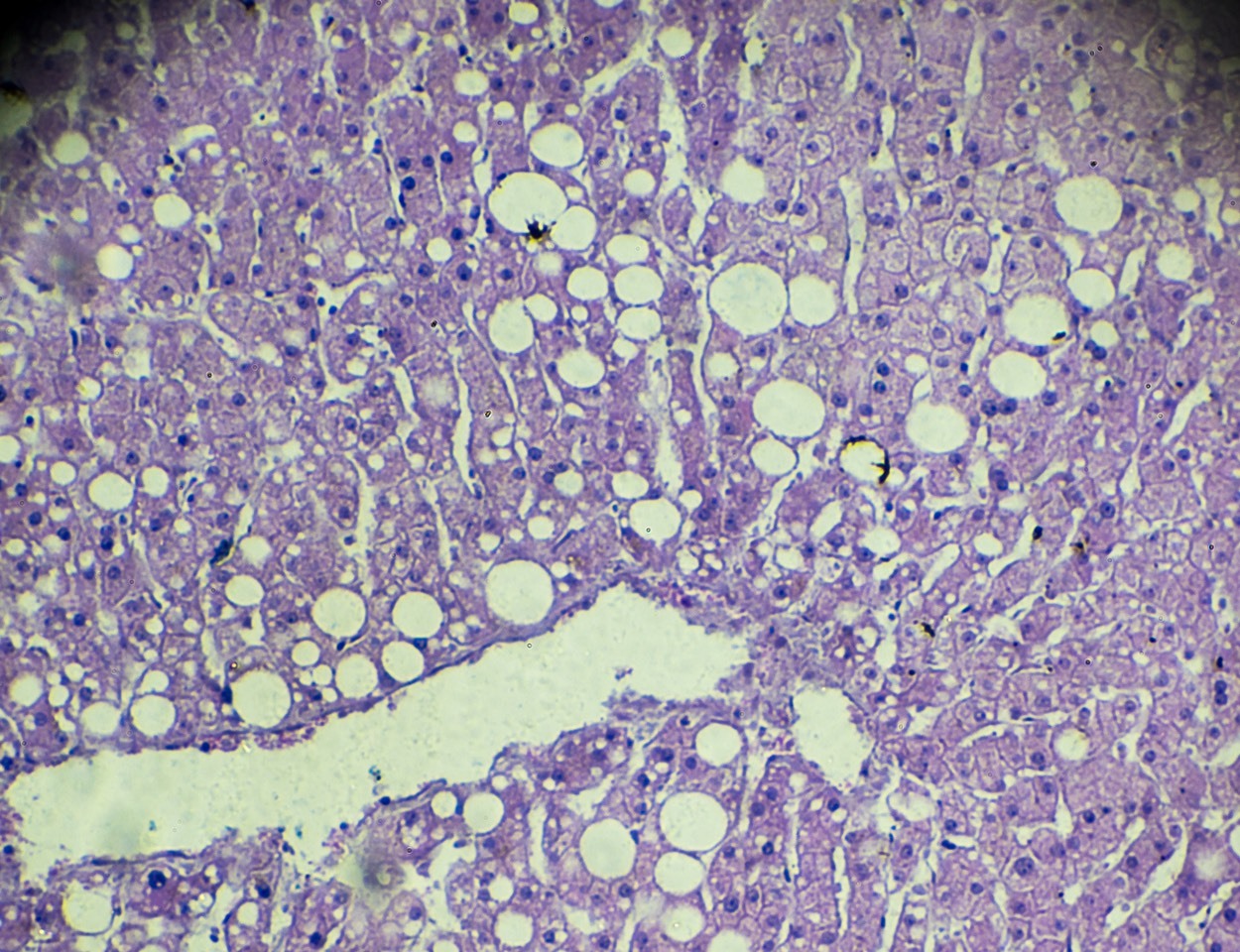
According to the American Liver Foundation, roughly 5 percent of U.S. adults — or 16.5 million people — are affected by a type of liver disease called NASH: non-alcoholic steatohepatitis. Characterized by buildup of fatty tissue in the liver, NASH continues to increase in prevalence alongside rising rates of obesity and type 2 diabetes. Unfortunately, NASH does not present symptoms until a patient is in the later stages of the disease; when left untreated, the disease can cause permanent damage. Currently, the disease can only be diagnosed through a painful, invasive and expensive liver biopsy. To properly diagnose NASH, and to help doctors identify patients at risk of dire health complications, better, less invasive and more reliable diagnostic tools are needed.
In 2019, the FNIH Biomarkers Consortium launched the Non-Invasive Biomarkers of Metabolic Liver Disease (NIMBLE) project to replace the current standard for diagnosing NASH with a patient-friendly, non-invasive approach. The NIMBLE team will compare promising new imaging-based and blood-based biomarkers individually and in combination against liver biopsies to identify a reliable, widely deployable, non-invasive marker to more easily diagnose NASH and monitor patient responses to treatments. Armed with this knowledge, physicians for the first time will be able to identify more effectively those patients who are most likely to progress to serious complications like liver failure or cancer.
“There is a broad consensus on the need to validate safe and simple approaches to replace biopsy for diagnosis and staging, and as a way to monitor the liver health of patients with NASH over time,” says Gail Cawkwell, of partner Intercept Pharmaceuticals. “We will get there faster if academic institutions, patient groups, drug developers, regulators and public health agencies all work together to pool our data, resources and knowledge.” Morris J. Birnbaum, of partner Pfizer, says, “NIMBLE has the potential to galvanize the entire field in our pursuit of an accurate, scalable, non-invasive tool for the diagnosis and staging of NASH.”
A breakthrough discovery in the diagnosis and monitoring of NASH is an essential goal for the research community, potentially helping patients with this deadly, yet invisible, disease.
